What is Signatera™?
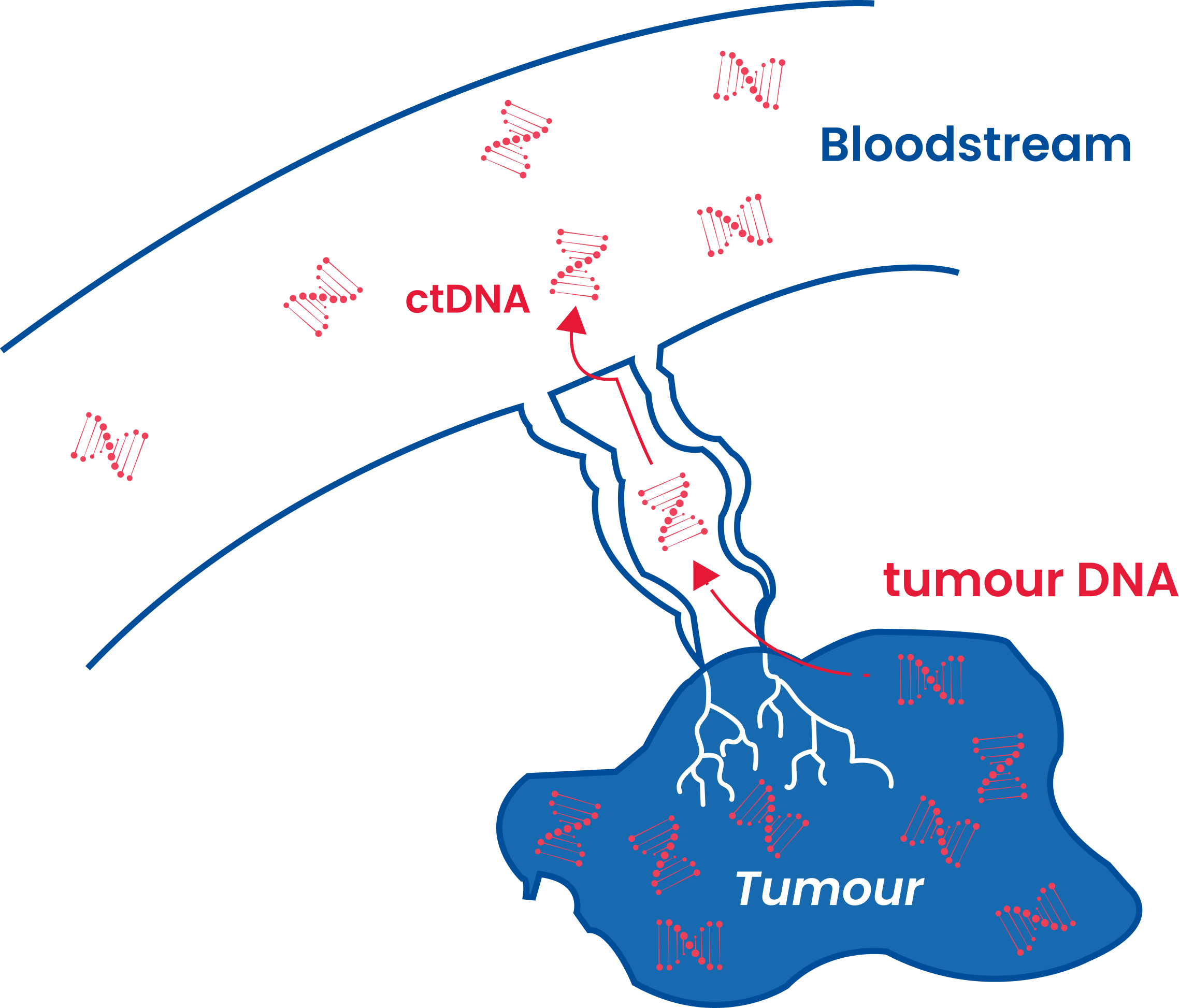
Each patient’s cancer is as unique as their fingerprint. Signatera™ is a custom-built, highly sensitive test that uses tumour and blood samples to detect very low levels of molecular residual disease (MRD), or small traces of cancer, using circulating tumour DNA (ctDNA), allowing an individual and their healthcare provider access to more information, sooner.
Signatera™ can identify whether cancer is still present or recurring earlier than traditional tools. A healthcare provider may order Signatera™ along with routine follow-up to determine:
- Signs of cancer remaining in the body
- If treatment (e.g., chemotherapy, radiation) is working
- Whether the cancer is recurring
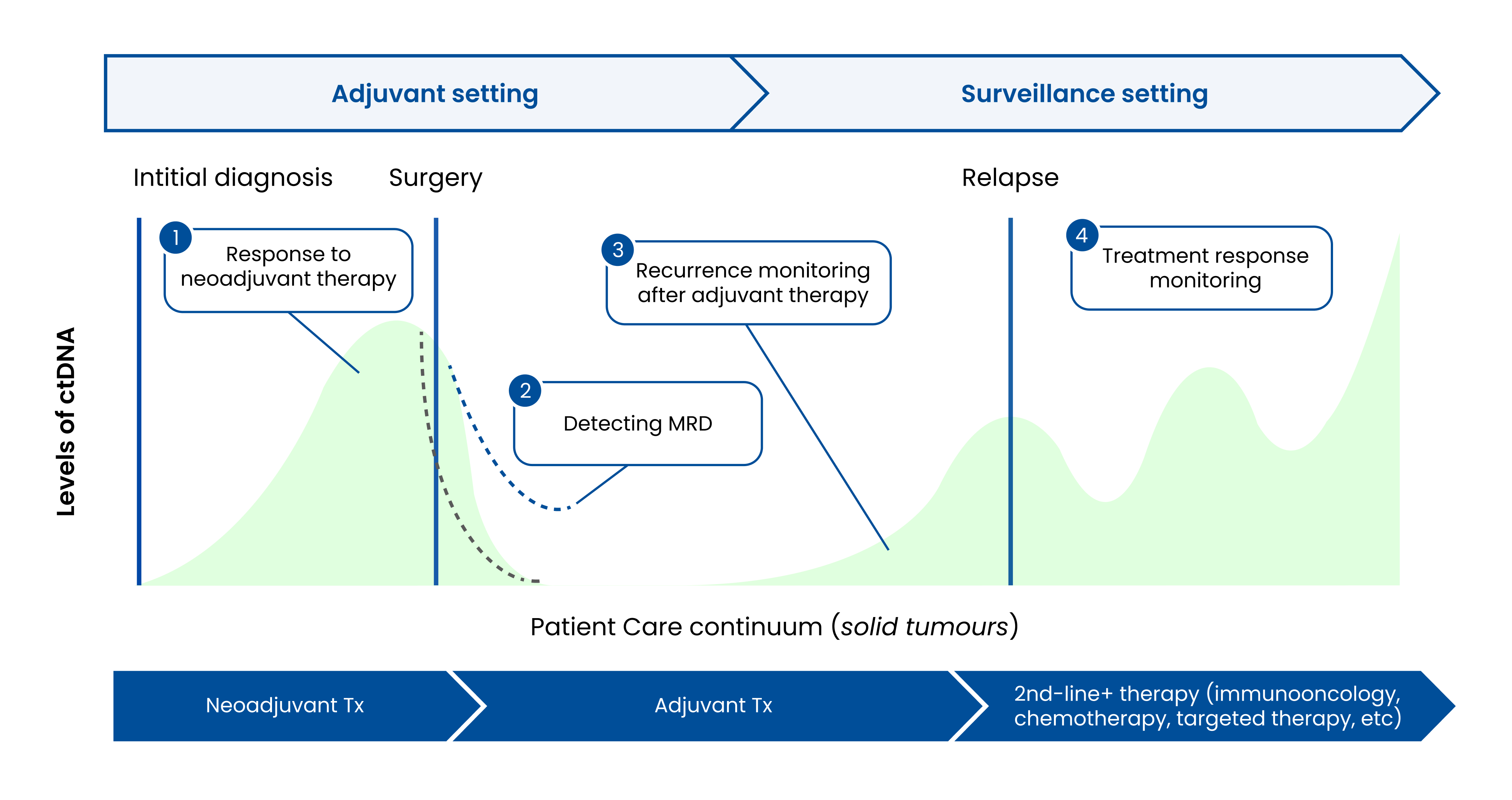
How Signatera™ helps patients through their treatment?
Living scan to scan can sometimes create anxiety for patients.
The question of relapse looms in the back of the mind:
Did the treatment work? Is the cancer coming back?
Through shared decision making, patients can work closely with their care team to incorporate Signatera into their treatment plan to provide additional information for confident decision making.
What does Signatera™ tell me?
Individuals who have been diagnosed with solid tumours (e.g., Colorectal, breast, ovarian, lung, bladder, melanoma) or those diagnosed with cancer (solid tumours) being treated with immunotherapy, and are seeking answers to the following questions:
- Is there cancer left in the body?
- Is additional treatment beneficial?
- Is the treatment working?
SignateraTM is not currently recommended for leukemias, non-solid-mass lymphomas, and cancer behind the blood-brain barrier such as CNS tumours.
Knowing early if there are traces of cancer present in the body can help the doctor or oncologist decide whether:
- An individual is responding to treatment
- Further cancer treatment needs to be considered
- There are signs that cancer has returned or progressed
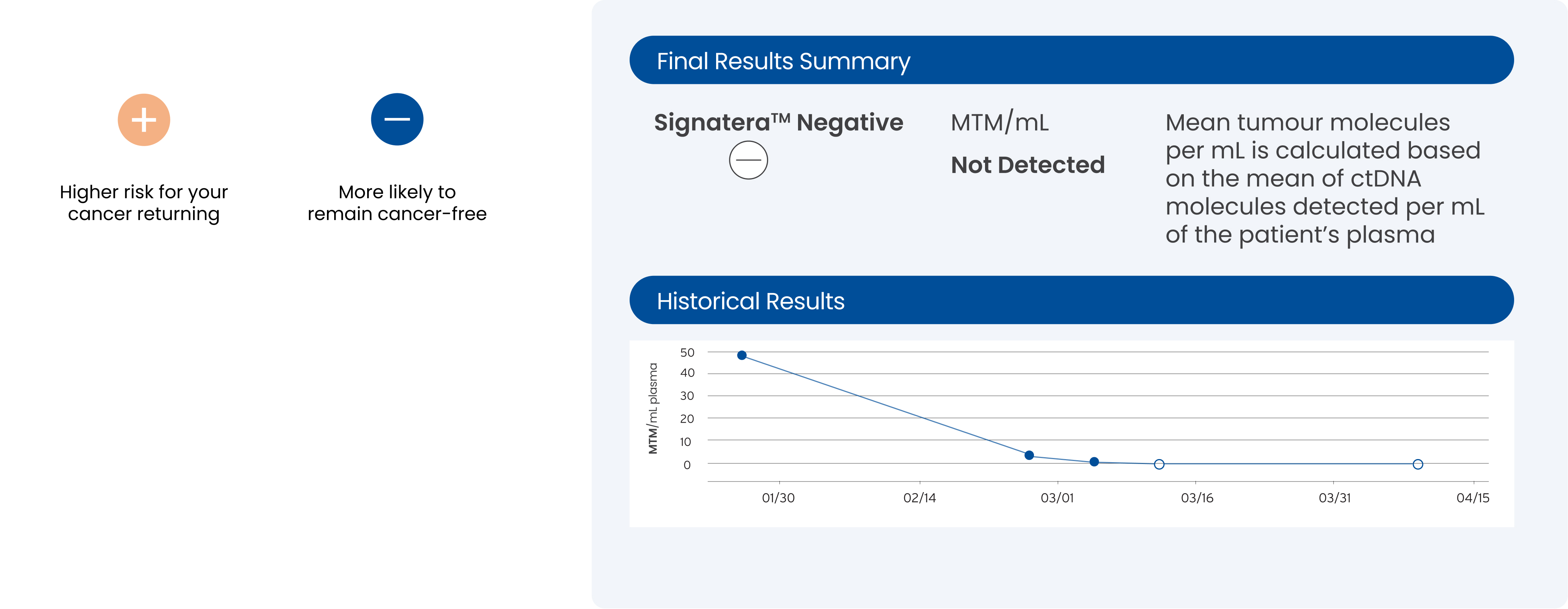
SignateraTM is a custom-designed test that is based on each patient’s unique set of tumor mutations. Knowing earlier if your cancer is likely to recur or has progressed after treatment can help you have a more informed discussion with your doctor on how to continue to treat or to detect changes in your disease.

One-time analysis
A one-time analysis of both blood and tissue determines your unique set of tumor mutations.

Signatera™ test custom-built
A SignateraTMtest is custom-built and personalized for you.
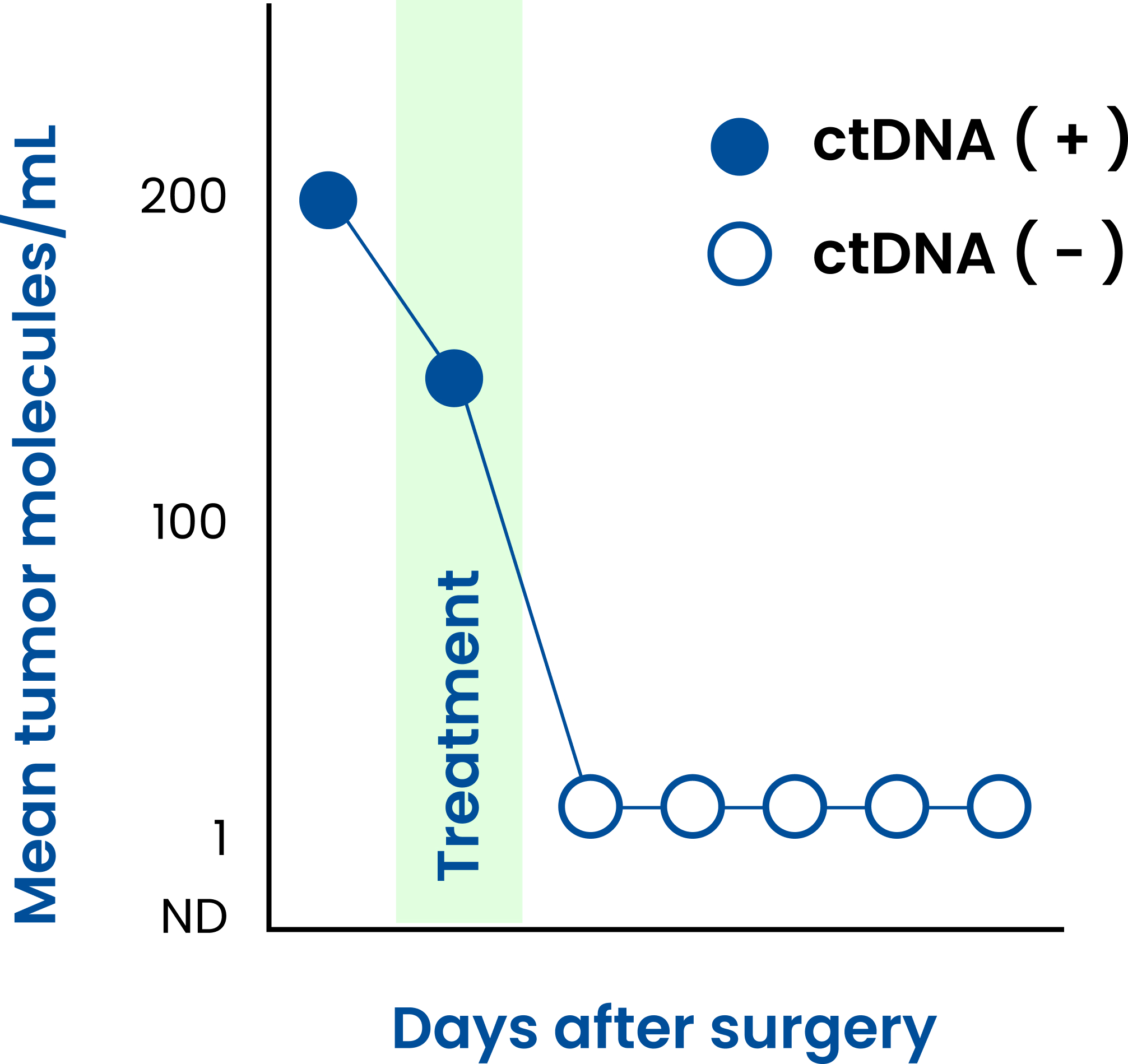
ctDNA levels monitored
SignateraTM detects the increase or decrease of ctDNA each time it is ordered as part of your routine follow-up blood tests.
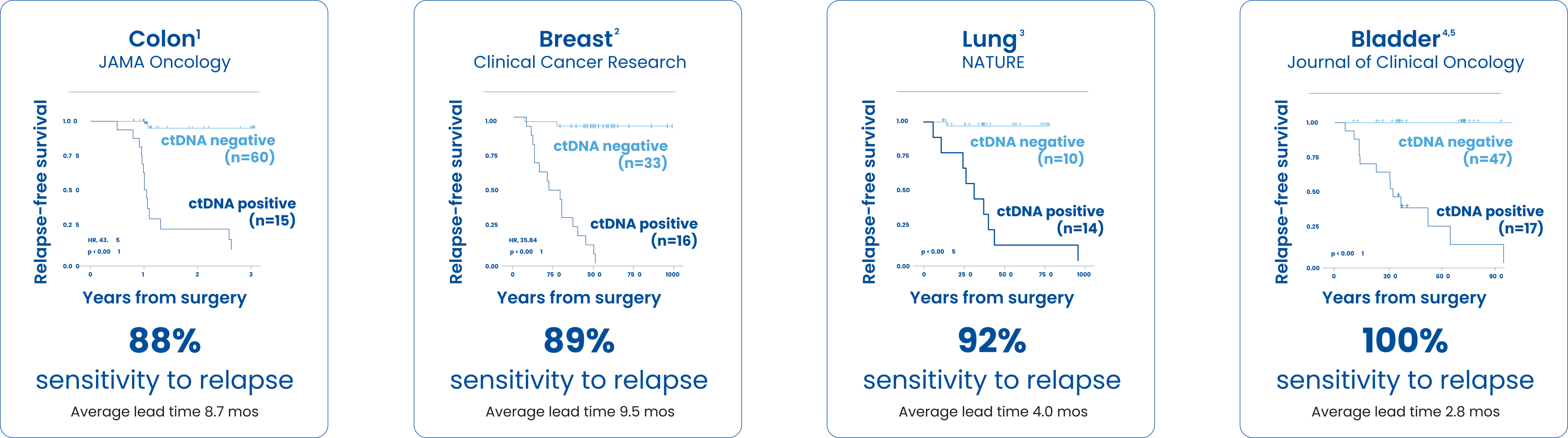
A positive SignateraTM result predicts relapse with an overall positive predictive value of more than 98%1-4
References
1Reinert T, Henriksen TV, Christensen E, et al. Analysis of Plasma Cell-Free DNA by Ultradeep Sequencing in Patients With Stage I to III Colorectal Cancer. JAMA Oncology. 2019;5(8):1124-1131.
2Coombes C, Page K, Salari R, et al. Personalized Detection of Circulating Tumor DNA Antedates Breast Cancer Metastatic Recurrence. Clinical Cancer Research. 2019;25(14):4255-4263.
3Abbosh C, Birkbak N, Wilson GA, et al. Phylogenetic ctDNA analysis depicts early-stage lung cancer evolution. Nature. 2017,545:446–451.
4Christensen E, Birkenkamp-Demtröder K, Sethi H, et al. Early Detection of Metastatic Relapse and Monitoring of Therapeutic Efficacy by Ultra-Deep Sequencing of Plasma Cell-Free DNA in Patients with Urothelial Bladder Carcinoma. 2019; 37(18):1547-1557. 5Data on file.
- Initial Test: 4-6 weeks*
- Subsequent Test: 1-2 weeks*
* In rare instances, turnaround times may be extended and/or require resubmissions of blood or tissue samples. The ordering healthcare provider will be notified in these instances. Turnaround times do not account for the time it takes the pathology lab to send the tumour sample; however, LifeLabs makes every attempt to expedite this process.
SignateraTM is recommended for periodic use over the course of your treatment as directed by your doctor to detect the presence of disease.